Abstract
The Cretaceous–Palaeogene mass extinction around 66 million years ago was triggered by the Chicxulub asteroid impact on the present-day Yucatán Peninsula1,2. This event caused the highly selective extinction that eliminated about 76% of species3,4, including all non-avian dinosaurs, pterosaurs, ammonites, rudists and most marine reptiles. The timing of the impact and its aftermath have been studied mainly on millennial timescales, leaving the season of the impact unconstrained. Here, by studying fishes that died on the day the Mesozoic era ended, we demonstrate that the impact that caused the Cretaceous–Palaeogene mass extinction took place during boreal spring. Osteohistology together with stable isotope records of exceptionally preserved perichondral and dermal bones in acipenseriform fishes from the Tanis impact-induced seiche deposits5 reveal annual cyclicity across the final years of the Cretaceous period. Annual life cycles, including seasonal timing and duration of reproduction, feeding, hibernation and aestivation, vary strongly across latest Cretaceous biotic clades. We postulate that the timing of the Chicxulub impact in boreal spring and austral autumn was a major influence on selective biotic survival across the Cretaceous–Palaeogene boundary.
Main
The Cretaceous-Palaeogene (K–Pg) mass extinction event affected biodiversity with high but poorly understood taxonomic selectivity. Among archosaurs, for example, all pterosaurs and non-avian dinosaurs succumbed in the K–Pg mass extinction, while crocodilians and birds survived into the Palaeogene period3,4. Direct consequences of the impact, including impact glass fallout, large-scale forest fires and tsunamis, are geologically documented more than 3,500 km from the Chicxulub impact crater5,6,7,8. Although direct effects of the impact devastated a vast geographical area, the global mass extinction probably unfolded during its aftermath, which involved rapid climatic deterioration estimated to have lasted up to several thousands of years9,10,11. Whether seasonal timing of the onset of these marked changes affected the selectivity of the K–Pg extinction could not yet be established owing to the lack of suitable records.
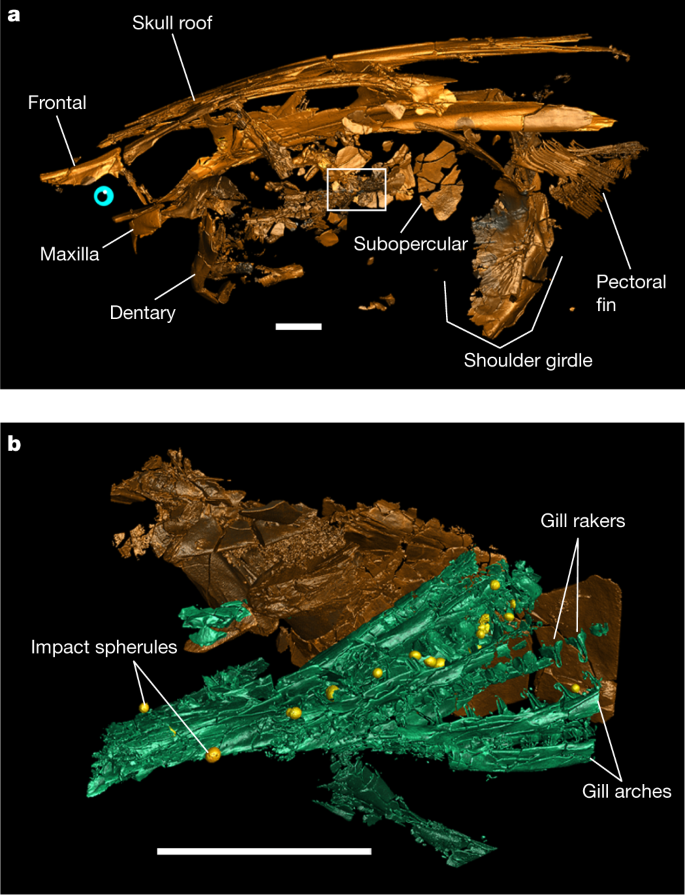
The Tanis event deposit in North Dakota (USA) is an exceptional seiche deposit preserving a rich thanatocoenosis (that is, a mass death assemblage) of latest Cretaceous biota at the top of the Hell Creek Formation. The majority of macrofossils encountered at the Tanis locality represent direct casualties of the K–Pg bolide impact that were buried within the impact-induced seiche deposit5. Tens of minutes after the impact, the seiche agitated large volumes of water and soil in the estuary of the Tanis river5. As the seiche proceeded upstream, it advected bones, teeth, bivalves, ammonites, benthic foraminifera (Extended Data Fig. 1a–c) and plant matter in the suspended load while impact spherules rained down from the sky5. Within the thanatocoenotic accumulation, abundant acipenseriforms—sturgeons and paddlefishes—became oriented along the seiche flow directions and buried alive with numerous impact spherules in their gills5 (Fig. 1, Extended Data Fig. 2a, b).
a, Three-dimensional rendering of paddlefish FAU.DGS.ND.161.4559.T in left lateral view with the location of a higher-resolution scan (depicted in b) indicated (white outline). b, Three-dimensional rendering of the subopercular and gills in a with trapped impact spherules (yellow). Scale bars, 2 cm. Two-dimensional tomographic data and fully annotated three-dimensional renderings are provided in Extended Data Fig. 2. A three-dimensional animated rendering of FAU.DGS.ND.161.4559.T is provided as Supplementary Video 1.
During the Maastrichtian (that is, the last age of the Cretaceous), the climate of present-day North Dakota involved four seasons that were documented in tree-ring records recovered from other Upper Cretaceous sites in the Hell Creek Formation12,13. Tanis was located at approximately 50° N during the latest Cretaceous and experienced distinct seasonality in rainfall and temperature14. Regional air temperatures were reconstructed to range from 4–6 °C in winter up to an average of about 19 °C in summer13,14. To uncover the season of the K–Pg bolide impact, we analysed osteohistological records of acipenseriform bone apposition in three paddlefish dentaries and three sturgeon pectoral fin spines that were excavated at the Tanis site in 2017 (Extended Data Fig. 1d–j). These skeletal elements preserve unaltered growth records from embryonic development up to death, making them highly suitable for life history reconstructions15,16.
Growth records of end-Cretaceous fishes
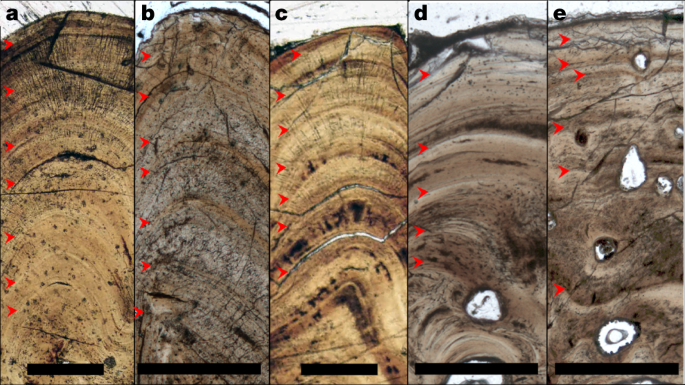
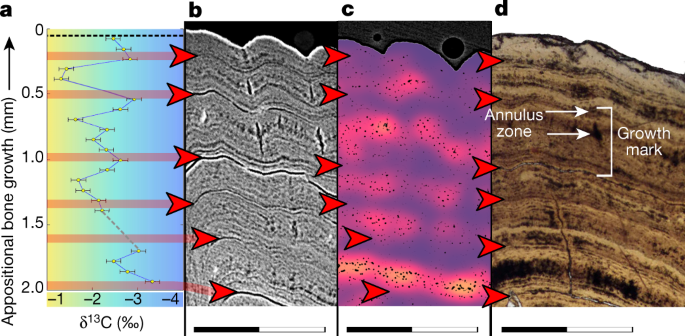
To trace appositional growth and pinpoint the season in which bone apposition terminated, we first assessed the preservation of bone growth patterns across the studied specimens. We prepared dermal bone slices of six acipenseriform specimens as microscopic slides and subjected these to osteohistological assessment, during which lines of arrested growth (LAGs) were easily recognized (Fig. 2). To corroborate the annual nature of the LAGs using virtual high-resolution osteohistology17,18, three-dimensional (3D) volumes were produced with propagation phase-contrast synchrotron radiation micro-computed tomography19 on beamline BM05 of the European Synchrotron Radiation Facility, France. The 3D nature of the synchrotron data enables optimal projection of the bone deposition pattern across multiple cross-sectional planes and resolved the exact relationship between seasonality and cyclical bone apposition in superb detail20. In addition, virtual osteohistology allowed us to visualize the seasonal fluctuations of osteocyte lacunar density and volume, which are poorly expressed in the physical 2D thin sections18 (Fig. 3c, d) . The osteohistological data (Figs. 2, 3, Extended Data Figs. 3–6) were complemented with an incremental carbon isotope record extracted from one of the paddlefish dentaries (VUA.GG.2017.X-2724).
a–e, Thin sections in transmitted light of VUA.GG.2017.MDX-3 (a), VUA.GG.2017.X-2743M (b), VUA.GG.2017.X-2744M (c), VUA.GG.2017.X-2733A (d) and VUA.GG.2017.X-2733B (e), showing congruent pacing of bone apposition during the final years of life, terminating in spring. Red arrows indicate LAGs. Scale bars, 0.5 mm.
a, 𝛿13C record expressed as ‰ on the Vienna Pee Dee Belemnite (VPDB) reference scale. The colour gradient highlights the theoretical range between maximum values during seasonal (summer) trophic increase of 13C (yellow) and minimum values during trophic decrease of 13C (winter) (blue). b, Virtual thick section (average-value projection with 0.1-mm depth) showing growth zones during the favourable growth seasons and annuli and LAGs outside the favourable growth seasons. c, Cell density map51 of a virtual thick section (minimum-value projection with 0.2-mm depth) showing fluctuating osteocyte lacunar densities and sizes, with higher densities and largest sizes recorded during the favourable growth seasons (orange) and lower densities and smaller sizes outside the favourable growth seasons (purple). A comparative image of a larger section of bone with scale is provided in Extended Data Fig. 6. d, Microscopic thin section in transmitted light showing LAGs (red arrows) and a single growth mark indicated (bracket) spanning the distance between two subsequent LAGs and including a zone and an annulus (Extended Data Fig. 10b). Scanning data visualized in b and c were obtained approximately 10-mm distal from the physically sectioned thin slice of d, which itself was located directly proximal to the thick section sampled for a. Scale bars, 1 mm. Corresponding osteohistological data of the other five sampled acipenseriform fishes are presented in Extended Data Figs. 3–5.
The tomographic data show that impact spherules associated with the paddlefish skeleton are present exclusively in its gill rakers5 and are absent elsewhere in the preserved specimen (Fig. 1). The absence of impact spherules outside the gill rakers demonstrates that spherules were filtered out of the surrounding waters but had not yet proceeded into the oral cavity or further down the digestive tract, and had not impacted the fish carcases during perimortem exposure. Impact spherule accumulation in the gill rakers and the arrival of the seiche waves must therefore have occurred simultaneously5, which implies that the acipenseriforms were alive and foraging during the bolide impact and the last minutes of the Cretaceous.
Well-conserved bone growth archives
The degree of preservation of the sampled acipenseriform bones was assessed using micro-X-ray fluorescence (Methods, Extended Data Figs. 7–9), which would reveal potential taphonomic elemental exchange that may have affected the primary stable isotope composition. The micro-X-ray fluorescence maps show that Fe and Mn oxides are present in the bone vascular canals and surrounding sediments (Extended Data Fig. 8), but have not invaded the bone apatite (Ca5(PO4,CO3)3(OH,F,Cl)). Detrital components, characterized by high concentrations of K and Si, remain restricted to the sediment matrix (Extended Data Fig. 8f–j). The bone apatite conserves a highly homogeneous distribution of P and Ca (Extended Data Fig. 9), which corroborates the unaltered preservation of these apatitic tissues. Skeletal remains of the paddlefishes and sturgeons thus experienced negligible diagenetic alteration, probably as a consequence of rapid burial and possibly aided by early Mn and Fe oxide seam formation21,22. The exquisite 3D preservation of delicate structures, including non-ossified tissues that originally enveloped the brain (Extended Data Fig. 2c–f), further demonstrates the excellent preservation of the fossils and absence of taphonomic reorganization23.
Consistent records of a spring death
Paddlefish dentaries form through perichondral ossification around the Meckel’s cartilage24. Sturgeon pectoral fin spines consist of dermal bone—an intramembranous skeletal tissue that forms in the mesenchyme (mesodermal embryonic tissue)25. Unlike endochondral bone, perichondral and dermal bone do not originate through mineralization of cartilaginous precursors26,27,28 but grow exclusively through incremental bone matrix apposition by secretion of a row of osteoblasts24,26,27,28. The thickness of one annual growth mark cumulatively spans a thick (favourable) growth zone, a thinner (slowly deposited) annulus and, ultimately, a LAG20. Our microscopic and virtual osteohistological data consistently show that the six fishes perished (that is, stopped growing) while forming a growth zone shortly after a LAG was deposited (Figs. 2, 3, Extended Data Figs. 3–6), which coincides with an early stage of the favourable growth season20. The outermost cortices of all six acipenseriform individuals studied here also exhibit increasing osteocyte lacunar densities and sizes towards their periosteal surfaces (Fig. 3c, Extended Data Figs. 5, 6). In all specimens, this density remained lower than the highest densities and average sizes recorded in previous years (Fig. 3c, Extended Data Figs. 3–6, 10b). As osteocyte lacunar density and size patterns were consistently cyclical across the preceding years during which they peaked at the climaxes of the growth seasons, the last recorded growth season had thus not yet climaxed at the time of death (Figs. 2, 3, Extended Data Figs. 3–6, 10b).
The inferred annual growth cycles are independently corroborated by a stable carbon isotope (𝛿13Csc) archive that recorded several years of seasonal dietary fluctuations in growing bone. Paddlefish VUA.GG.2017.X-2724 also yielded, in addition to this 𝛿13Csc archive, an oxygen isotope (𝛿18Osc) record across the final six years of its life (Supplementary Data Table 1, Extended Data Fig. 10a, Methods). The low and constant 𝛿18Osc values in VUA.GG.2017.X-2724 reflect exclusive inhabitation of freshwater environments by the paddlefishes. This implies that their osteohistological records must have captured seasonal variability rather than, for example, migration between saline and freshwater habitats. Although modern sturgeons are known to have anadromous lifestyles29,30, this remains to be confirmed for the fossil sturgeons at Tanis, as isotopic data from sturgeon pectoral fin spines could not be secured (Methods, ‘Micromill’). Notably, the osteohistological records of all our sturgeons and paddlefishes converge on the same annual growth phase, despite their potential different lifestyles.
Like their modern-day relatives, the latest Maastrichtian paddlefishes of Tanis were filter feeders that presumably consumed copepods and other zooplankton29,30,31. These fishes probably experienced an annual feeding pattern, determined by fluctuating food availability, that peaked between spring and autumn31. During maximum productivity, ingested zooplankton enriches the growing skeleton of filter-feeding fishes with 13C relative to 12C32,33. Thus, the cyclically elevated 13C/12C ratios in paddlefish VUA.GG.2017.X-2724 (Fig. 3a) reflect distinct episodes of high food availability and consumption. Carbon isotope records across the growth record of Paddlefish VUA.GG.2017.X-2724 indicate that peak annual growth rate was not yet attained and the feeding season had thus not yet climaxed—corroborating a boreal spring death.
Implications for selective K–Pg survival
The Chicxulub bolide impact caused a global heat pulse that ignited widespread wildfires9,34. After this heat wave, the last boreal spring of the Mesozoic transitioned to a global impact winter10. Although a June timing for the K–Pg impact has been suggested on the basis of palaeobotanical indications for anomalous freezing in this region (Wyoming, USA)35, the palaeobotanical identities, taphonomic inferences and stratigraphic assumptions underlying that conclusion have since all been refuted36,37,38,39. Moreover, post-impact cooling happened in the first months to decades following the K–Pg impact10, which renders proxies registering post-impact freezing conditions asynchronous with the impact event itself.
A suite of impact-induced phenomena contributed to the K–Pg extinction on differing timescales40,41. In the days to months following the impact, its instantaneous effects, such as intense infrared radiation caused by ejecta reentry34, resulting wildfires9,34 and the spread of sulfurous aerosols leading to acid precipitation42 must have predominantly afflicted the exposed continental environments. Although negotiating these hostile conditions would not have guaranteed survival, an early clade-wide eradication would always have meant immediate extinction41.
The seasonal timing of the catastrophic end-Cretaceous bolide impact places the event at a particularly sensitive stage for biological life cycles in the Northern Hemisphere. In many taxa, annual reproduction and growth take place during spring. Species with longer incubation times, such as non-avian reptiles, including pterosaurs and most dinosaurs, were arguably more vulnerable to sudden environmental perturbations than other groups43 (for example, birds). Southern Hemisphere ecosystems, which were struck during austral autumn, appear to have recovered up to twice as fast as Northern Hemisphere communities44, consistent with a seasonal effect on biotic recovery.
Subterranean sheltering conceivably contributed to the cynodont survival of the Permo-Triassic (PT) crisis45. Similarly, large-scale wildfires raging across the Southern Hemisphere9,34,41 may have been evaded by hibernating mammals that were already sheltered in burrows34,41 in anticipation of austral winter. Additional modes of seasonal dormancy, torpor and/or aestivation, which are nowadays practised by various mammals46,47 as well as certain amphibians, birds and crocodilians48, could have facilitated further underground survival. In the aftermath of the K–Pg event, ecological networks collapsed from the bottom up. Floral necrosis9 and extinction immediately affected species dependent on primary producers, while some animals capable of exploiting alternative resources—for example, certain birds and mammals49,50—persisted.
Conclusions
Seasonal timing of the Chicxulub impact in boreal spring and austral autumn will aid in further calibrating evolutionary models exploring the selectivity of the K–Pg extinction and the asymmetry in extinction and recovery patterns between the two hemispheres. Decoupling short- and long-term effects of the bolide impact on the K–Pg mass extinction will also aid in identifying extinction risks and modes of ecological deterioration caused by the forthcoming global climate change. The uniquely constrained Tanis site5 offers valuable proxies for reconstructing the environmental, climatological and biological conditions that prevailed locally when the Mesozoic ended.
Methods
Fieldwork
Excavation at the Tanis locality in south-western North Dakota took place between 10 August and 20 August 2017. Sections of dentaries of paddlefishes and pectoral fin spines of sturgeons were collected in the field for histological study.
Thin sectioning
Four out of the six samples were excavated from the sediment matrix. These included all sturgeon pectoral fin spines (VUA.GG.2017.X-2743M, VUA.GG.2017.X-2744M, and VUA.GG.2017.MDX-3) and one of the paddlefish dentaries (VUA.GG.2017.X-2724). Paddlefish dentaries VUA.GG.2017.X-2733A and VUA.GG.2017.X-2733B, belong to two individuals, were uncovered aligned to each other and fractured upon discovery. To avoid further damage, the samples were embedded in epoxy resin prior to thin sectioning. All specimens were cut with a diamond saw and polished to obtain microscopic thin sections (about 50-μm thick) and thick sections for micromilling (about 200-μm thick). See Extended Data Fig. 1e–j for images of the specimens and the sampling locations.
Osteohistological analysis
In the acipenseriform dermal bones examined in this study, annual growth cyclicity can be traced through growth marks (GMs).
A GM spans a single growth cycle that typically lasts one year and can be divided into a zone, an annulus, and a LAG20,52. The zone is deposited during a period of relative rapid growth in the active or favourable growth season20. The annulus is subsequently formed when growth slows down towards the end of the growth season20. Finally, a LAG forms when growth periodically ceases until the next growth season starts and a new zone is deposited20.
During the formation of a growth zone, the density and volumes of osteocyte lacunae (OL; subcircular dark features in Extended Data Fig. 10a) initially increase when growth accelerates. Subsequently, towards and into the annulus, OL density and volume decrease as growth slows down18. Because a LAG coincides with a temporary arrest of local osteogenesis, it is only expressed when deposition of a new growth zone has commenced. All six studied specimens show a LAG relatively close to the outermost partial growth zone.
In fossil bone, LAGs often appear as sharply defined dark lines53 that typically constitute a poorly coherent interface between adjacent bone layers, thus facilitating (local) delamination between adjacent cortical layers53. During fossilization, percolation products can accumulate in these gaps and thereby (locally) accentuate the LAGs51,53 (figure 31.3G of ref. 52). Based on this well-understood expression of LAGs (that we recognize from our own experience as well; S.S. personal observation), we have consistently identified the LAGs as locally stained dark lines that may be associated with circumferentially propagated cracked surfaces which are oriented parallel to the periosteal deposits.
Besides cyclical seasonal factors that synchronize GM accretion, stress may induce additional diapause stages that result in supplementary marks within a single year54. Cessation of growth for the duration of several weeks can provoke the formation of a LAG54. However, such non-cyclical marks “tend to be haphazard rather than regular (that is, they do not reflect a particular spacing or rhythm)” and do not encircle the cortex of the skeletal element but “tend to be locally confined to an arc”55.
As the studied bones yield only regularly spaced GMs along their complete circumference, we confidently identify the preserved GMs as annual cycles. Moreover, the fluctuating quantified density and volumes of osteocyte lacunae (Extended Data Fig. 6d–f) and the carbon isotopic record (Fig. 3a, Extended Data Fig. 10a) across the final seven years of growth of VUA.GG.2017.X-2724 are exclusively consistent with the identification of annual LAGs in corresponding physical thin sections. In all studied specimens, bone growth terminated during the process of zonal bone growth.
Micro-X-ray fluorescence
Fragments of the paddlefish and sturgeon samples that remained after thin sectioning were analysed with microX-ray fluorescence. High-resolution elemental mapping was conducted using a Bruker M4 Tornado 2D spectrometer at 50 kV and 600 μA, without a filter, and at an acquisition rate of 20 μm per 5 ms at the Vrije Universiteit Brussel.
Micromill
The growth increments were sampled in the thick sections (about 200-μm thick) at the highest possible accuracy using a Micromill (Merkantek). Drill transects were assigned in the accompanying software and after each individual sample was collected, the drill bit was cleaned with ethanol. Not all thick sections were suitable for micromilling. The lobed anatomy of the sturgeon fin spines (VUA.GG.2017.X-2743M and VUA.GG.2017.X-2744M) proved too complex to reliably sample single growth increments with the micromill. Paddlefish dentaries VUA.GG.2017.X-2733A and VUA.GG.2017.X-2733B only exposed a few growth lines that were too narrow to sample with the micromill. Sturgeon pectoral fin spine VUA.GG.2017.MDX-3 and paddlefish dentary VUA.GG.2017.X-2724 were sampled up to the outermost growth increment.
Stable isotope analysis
Micromilled hydroxyapatite samples of specimen VUA.GG.2017.X-2724 weighing about 50 μg were placed in Exetainer vials (Labco) and flushed with purified helium gas. For reference, the analysed amounts of structural carbonate are equivalent to anout 5 μg of CaCO3. Orthophosphoric acid was subsequently added and allowed to react for 24 h at 45 °C. VUA.GG.2017.MDX-3 was routinely analysed with a Thermo Finnigan Deltaplus mass spectrometer connected to a Thermo Finnigan GasBench II at the Earth Sciences Stable Isotope Laboratory (Vrije Universiteit, Amsterdam). However, the amount of CO2 generated was found to be too small to permit reliable isotopic determinations. To alleviate this, the GasBench was provisionally interfaced with a cold trap in which the CO2 was frozen with liquid nitrogen during a 2 min period. After trapping for 2 min, an accurate single-pulse measurement was performed for each of the apatitic samples and standards. Each isotopic sample determination was preceded by six pulses of monitoring CO2 with a calibrated isotopic composition to assure stable conditions of the mass spectrometer. The isotopic measurements of the weighted micromilled samples were bracketed by the analyses of the inter-laboratorial apatite standard (Ag-Lox) to account for the linearity effect56. After corrections, the uncertainties for 𝛿13C and 𝛿18O of the Ag-Lox (n = 4) were 0.16 ‰ and 0.39 ‰ (1 s.d.) respectively. Although the amount of extracted and analysed structural carbonate remains insufficient for optimal isotopic determination, the relatively large recovered 𝛿13C variability still yields a meaningful record across the appositional bone archive. The 𝛿18O values of structural carbonate, unlike those of phosphate (PO4)57, do not offer a sensitive palaeo-environmental proxy for accurate seasonal temperature reconstructions58. However, the relatively constant 𝛿18O values of structural carbonate precludes large 𝛿18O changes in ambient water, such as shifts between freshwater and saline environments.
Propagation phase-contrast synchrotron radiation micro-computed tomography
Paddlefish specimen FAU.DGS.ND.161.4559.T lacks the paddle-shaped rostrum and all aspects caudal to the pectoral girdle. FAU.DGS.ND.161.4559.T was provided by the Palm Beach Museum of Natural History. Data acquisition took place in May 2018 on Beamline BM05 of the European Synchrotron Radiation Facility, Grenoble, France59. The complete specimen was scanned at an average energy of 132 keV using the white beam of BM05 filtered with 0.4 mm of Mo and 9 mm of Cu. The detector was composed of a 2-mm-thick LuAG:Ce scintillator optically coupled to a PCO edge 4.2 CLHS sCMOS camera. The resulting voxel size was 43.5 µm. To obtain sufficient propagation phasecontrast, the distance between the sample and the detector was set at 5 m. A total of 205 scans, each consisting of 5,000 projections taken at 7-ms intervals, were performed with a vertical displacement of 1.4 mm at a vertical field of view of 2.8 mm to ensure a double scan of the complete samples. Scans were performed in half-acquisition mode to enlarge the lateral field of view. The volume was reconstructed using a single-distance phase retrieval algorithm coupled with filtered back projection as implemented in the ESRF software PyHST2. Vertical concatenation, 16-bit conversion, and ring artefact corrections were performed using MATLAB scripts developed in-house. The gill region and impact spherules were subsequently scanned at a voxel size of 13.67 μm (filters: 0.4 mm of Mo and 6 mm of Cu, scintillator: LuAG:Ce, 500-μm thick, detected energy: 166 keV, propagation distance: 2.5 m). The samples were scanned in half-acquisition mode in two columns of 77 scans, each consisting of 4,998 projections with exposure times of 0.05 s, that were laterally concatenated after reconstruction. Finally, sample (VUA.GG.2017.X-2724) from the paddlefish dentaries and (VUA.GG.2017.MDX-3, VUA.GG.2017.X-2743M and VUA.GG.2017.X-2744M) of the sturgeon pectoral fin spines were scanned at 4.35 µm voxel size for osteohistological analysis60 (filters: 3.5 mm of Al plus 11 bars Al with a diameter of 5 mm, scintillator: LuAG:Ce scintillator, 500-µm thick, detected energy: 92 keV, propagation distance: 1.5 m). The samples were scanned in half-acquisition mode in one single column of 22 scans, each consisting of 4,998 projections with exposure times of 60 ms.
Digital 3D extraction of the bones and impact spherules was performed in VGStudio MAX 3.2 (Volume Graphics). VGStudio MAX 3.2 furthermore enabled the creation of virtual thick sections of the osteohistological samples through the ‘thick slab-mode’, which captures the maximum, average, or minimum, grey-level values along the desired field depth. Virtual thick sections were obtained from the average grey-level values at a thickness of 100 µm following optimal 3D alignment of the annuli and LAGs. Additional virtual thick sections were created from the minimum grey-level values at a thickness of 200 µm to best resolve the sizes and distributions of osteocyte lacunae. A coloured map of the density of the osteocyte lacunar distribution was created with a Gaussian filter51. Finally, we visualized the annual cyclicity of osteocyte lacunar volumes18 in paddlefish dentary VUA.GG.2017.X-2724. As the resolution of our data (voxel size of 4.35 μm; appropriate for assessing GMs and osteocyte lacunar distributions) is sixfold lower than that used for earlier osteocyte lacunar volumetric quantification in fish bones18 (voxel size of 0.7 μm), our result should be considered with appropriate care. Closely spaced (large) osteocyte lacunae may occasionally be conjoined and additional phenomena in the broad size range of osteocyte lacunae may be incidentally included in the visualized distribution. Moreover, in tomographic data, osteocyte lacunae are delimited by slight colour gradients (rather than discrete lines) that scale with voxel size. Because the outermost feature fringe contributes disproportionally to recovered volumes, these values are somewhat skewed relative to the original osteocyte lacunar volumes, which likely produces exaggerated volume values. Therefore, although all rendered features were extracted with a single thresholding operation and relative patterns are conservatively retained, absolute volume values are best considered in a comparative context.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
All isotopic, geochemical, and osteohistological data are included in the paper and Extended Data. Tomographic data of FAU.DGS.ND.161.4559.T, VUA.GG.2017.X-2724, VUA.GG.2017.MDX-3, VUA.GG.2017.X-2743M, and VUA.GG.2017.X-2744M are available at https://doi.org/10.5281/zenodo.5776294 and the http://paleo.esrf.eu database.
References
-
Alvarez, L. W., Alvarez, W., Asaro, F. & Michel, H. V. Extraterrestrial cause for the Cretaceous–Tertiary extinction. Science 208, 1095–1108 (1980).
-
Smit, J. & Hertogen, J. An extraterrestrial event at the Cretaceous–Tertiary boundary. Nature 285, 198–200 (1980).
-
Raup, D. M. Biological extinction in earth history. Science 231, 1528–1533 (1986).
-
Schulte, P. et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous–Paleogene boundary. Science 327, 1214–1218 (2010).
-
DePalma, R. A. et al. A seismically induced onshore surge deposit at the KPg boundary, North Dakota. Proc. Natl Acad. Sci. USA 116, 8190–8199 (2019).
-
Smit, J. et al. Tektite-bearing, deep-water clastic unit at the Cretaceous–Tertiary boundary in northeastern Mexico. Geology 20, 99–103 (1992).
-
Alvarez, W. in The Cretaceous-Tertiary Event and Other Catastrophes in Earth History (eds Ryder, G. et al.) 141–150 (Geological Society of America, 1996).
-
Smit, J. The global stratigraphy of the Cretaceous–Tertiary boundary impact ejecta. Annu. Rev. Earth Planet. Sci. 27, 75–113 (1999).
-
Morgan, J., Artemieva, N. & Goldin, T. Revisiting wildfires at the K–Pg boundary. J. Geophys. Res. 118, 1508–1520 (2013).
-
Vellekoop, J. et al. Rapid short-term cooling following the Chicxulub impact at the Cretaceous–Paleogene boundary. Proc. Natl Acad. Sci. USA 111, 7537–7541 (2014).
-
Vellekoop, J. et al. Evidence for Cretaceous-Paleogene boundary bolide ‘impact winter’ conditions from New Jersey, USA. Geology 44, 619–622 (2016).
-
Golovneva, L. B. The Maastrichtian (Late Cretaceous) climate in the northern hemisphere. J. Geol. Soc. Lond. 181, 43–54 (2000).
-
Wolfe, J. A. & Upchurch Jr, G. R. North American nonmarine climates and vegetation during the Late Cretaceous. Palaeogeogr. Palaeocl. 61, 33–77 (1987).
-
Hallam, A. A review of Mesozoic climates. J. Geol. Soc. London 142, 433–445 (1985).
-
Adams, L. A. Age determination and rate of growth in Polyodon spathula, by means of the growth rings of the otoliths and dentary bone. Am. Midl. Nat. 28, 617–630 (1942).
-
Bakhshalizadeh, S., Bani, A., Abdolmalaki, S. & Moltschaniwskyj, N. Identifying major events in two sturgeons’ life using pectoral fin spine ring structure: exploring the use of a non-destructive method. Environ. Sci. Pollut. R 24, 18554–18562 (2017).
-
Sanchez, S., Ahlberg, P. E., Trinajstic, K. M., Mirone, A. & Tafforeau, P. Three-dimensional synchrotron virtual paleohistology: a new insight into the world of fossil bone microstructures. Microsc. Microanal. 18, 1095–1105 (2012).
-
Davesne, D., Schmitt, A. D., Fernandez, V., Benson, R. B. & Sanchez, S. Three‐dimensional characterization of osteocyte volumes at multiple scales, and its relationship with bone biology and genome evolution in ray‐finned fishes. J. Evol. Biol. 33, 808–830 (2020).
-
Tafforeau, P., Bentaleb, I., Jaeger, J.-J. & Martin, C. Nature of enamel laminations and mineralization in rhinoceros enamel using histology and X-ray synchrotron microtomography: potential implications for palaeoenvironmental isotopic studies. Palaeogeogr. Palaeocl. 246, 206–227 (2007).
-
Castanet, J. Bone—Volume 7: Bone Growth (ed. Hall, B. K.) 245–283 (CRC Press, 1993).
-
Hedges, R. E. Bone diagenesis: an overview of processes. Archaeometry 44, 319–328 (2002).
-
Dumont, M. et al. Synchrotron XRF analyses of element distribution in fossilized sauropod dinosaur bones. Powder Diffr. 24, 130–134 (2009).
-
Pradel, A. et al. Skull and brain of a 300-million-year-old chimaeroid fish revealed by synchrotron holotomography. Proc. Natl Acad. Sci. USA 106, 5224–5228 (2009).
-
Weigele, J. & Franz‐Odendaal, T. A. Functional bone histology of zebrafish reveals two types of endochondral ossification, different types of osteoblast clusters and a new bone type. J. Anat. 229, 92–103 (2016).
-
Enlow, D. H. The Human Face. An Account of the Postnatal Growth and Development of the Craniofacial Skeleton (Harper and Row, 1968).
-
Grande, L. & Bemis, W. E. Osteology and phylogenetic relationships of fossil and recent paddlefishes (Polyodontidae) with comments on the interrelationships of Acipenseriformes. J. Vert. Paleo. 11, 1–121 (1991).
-
De Ricqlès, A. J., Meunier, F. J., Castanet, J. & Francillon-Vieillot, H. Bone 3, Bone Matrix and Bone Specific Products (CRC Press, 1991).
-
Hall, B. K. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology (Elsevier, 2005).
-
Bemis, W. E. & Kynard, B. Sturgeon rivers: an introduction to acipenseriform biogeography and life history. Environ. Biol. Fish 48, 167–183 (1997).
-
LeBreton, G. T., Beamish, F. W. H. & McKinley, S. R. (eds) Sturgeons and paddlefish of North America, Vol. 27 (Springer, 2004)
-
Blackwell, B. G., Murphy, B. R. & Pitman, V. M. Suitability of food resources and physicochemical parameters in the lower Trinity River, Texas for paddlefish. J. Freshw. Ecol. 10, 163–175 (1995).
-
Fry, B. & Sherr, E. B. 𝛿13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Ecol. Stud. https://doi.org/10.1007/978-1-4612-3498-2_12 (1989).
-
Finlay, J. C. Stable‐carbon‐isotope ratios of river biota: implications for energy flow in lotic food webs. Ecology 82, 1052–1064 (2001).
-
Robertson, D. S., Lewis, W. M., Sheehan, P. M. & Toon, O. B. K–Pg extinction: reevaluation of the heat‐fire hypothesis. J. Geophys. Res. 118, 329–336 (2013).
-
Wolfe, J. A. Palaeobotanical evidence for a June ‘impact winter’ at the Cretaceous/Tertiary boundary. Nature 352, 420–423 (1991).
-
Nichols, D. J. Plants at the K/T boundary. Nature 356, 295–295 (1992).
-
Hickey, L. J. & McWeeney, L. J. Plants at the K/T boundary. Nature 356, 295–296 (1992).
-
McIver, E. E. Paleobotanical evidence for ecosystem disruption at the Cretaceous–Tertiary boundary from Wood Mountain, Saskatchewan, Canada. Can. J. Earth Sci. 36, 775–789 (1999).
-
Upchurch, G. R., Lomax, B. H. & Beerling, D. J. Paleobotanical evidence for climatic change across the Cretaceous–Tertiary boundary, North America: twenty years after Wolfe and Upchurch. Cour. Forsch. Senck 258, 57 (2007).
-
Kring, D. A. The Chicxulub impact event and its environmental consequences at the Cretaceous–Tertiary boundary. Palaeogeogr. Palaeocl. 255, 4–21 (2007).
-
Robertson, D. S., McKenna, M. C., Toon, O. B., Hope, S. & Lillegraven, J. A. Survival in the first hours of the Cenozoic. Geol. Soc. Am. Bull. 116, 760–768 (2004).
-
D’Hondt, S., Pilson, M. E., Sigurdsson, H., Hanson Jr, A. K. & Carey, S. Surface-water acidification and extinction at the Cretaceous–Tertiary boundary. Geology 22, 983–986 (1994).
-
Erickson, G. M., Zelenitsky, D. K., Kay, D. I. & Norell, M. A. Dinosaur incubation periods directly determined from growth-line counts in embryonic teeth show reptilian-grade development. Proc. Natl Acad. Sci. USA 114, 540–545 (2017).
-
Donovan, M. P., Iglesias, A., Wilf, P., Labandeira, C. C. & Cúneo, N. R. Rapid recovery of Patagonian plant–insect associations after the end-Cretaceous extinction. Nat. Ecol. Evol. 1, 0012 (2016).
-
Fernandez, V. et al. Synchrotron reveals Early Triassic odd couple: injured amphibian and aestivating therapsid share burrow. PLoS ONE 8, e64978 (2013).
-
Nowack, J., Cooper, C. E. & Geiser, F. Cool echidnas survive the fire. Proc. R. Soc. B 283, 20160382 (2016).
-
Lovegrove, B. G., Lobban, K. D. & Levesque, D. L. Mammal survival at the Cretaceous–Palaeogene boundary: metabolic homeostasis in prolonged tropical hibernation in tenrecs. Proc. R. Soc. B 281, 20141304 (2014).
-
Withers, P. C. & Cooper, C. in Encyclopedia of Ecology (eds Jorgensen, S. E. & Fath, B.) 952–957 (Elsevier, 2008).
-
Field, D. J. et al. Early evolution of modern birds structured by global forest collapse at the end-Cretaceous mass extinction. Curr. Biol. 28, 1825–1831 (2018).
-
Schleuning, M. et al. Ecological networks are more sensitive to plant than to animal extinction under climate change. Nat. Commun. 7, 13965 (2016).
-
Sanchez, S. et al. 3D microstructural architecture of muscle attachments in extant and fossil vertebrates revealed by synchrotron microtomography. PloS ONE 8, e56992 (2013).
-
de Buffrénil, V., Quilhac, A. & Castanet, J. in Vertebrate Skeletal Histology and Paleohistology (eds de Buffrénil, V. et al.) 626–645 (Routledge, 2021).
-
Lee, A. H. & O’Connor, P. M. Bone histology confirms determinate growth and small body size in the noasaurid theropod Masiakasaurus knopfleri. J. Vertebr. Paleontol. 33, 865–876 (2013).
-
Klevezal, G. A. & Stewart, B. S. Patterns and calibration of layering in tooth cementum of female northern elephant seals, Mirounga angustirostris. J. Mammal. 75, 483–487 (1994).
-
Woodward, H. N., Padian, K. and Lee, A. H. In Bone Histology of Fossil Tetrapods—Advancing Methods, Analysis and Interpretation (eds Padian, K. & E. T. Lamm) 195–215 (Univ. California Press, 2013).
-
Vonhof, H. B. et al. High‐precision stable isotope analysis of < 5 μg CaCO3 samples by continuous‐flow mass spectrometry. Rapid Commun. Mass Spectr. 34, e8878 (2020).
-
Pucéat, E. et al. Revised phosphate–water fractionation equation reassessing paleotemperatures derived from biogenic apatite. Earth Planet. Sci. Lett. 298, 135–142 (2010).
-
Vennemann, T. W., Hegner, E., Cliff, G. & Benz, G. W. Isotopic composition of recent shark teeth as a proxy for environmental conditions. Geochim. Cosmochim. Acta 65, 1583–1599 (2001).
-
Tafforeau, P. et al. Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl. Phys. A 83, 195–202 (2006).
-
Tafforeau, P. & Smith, T. M. Nondestructive imaging of hominoid dental microstructure using phase contrast X-ray synchrotron microtomography. J. Hum. Evol. 54, 272–278 (2008).
Acknowledgements
M.A.D.D. was partially funded by an EAVP Research Grant (ERG) awarded by the European Association of Vertebrate Palaeontologists. D.F.A.E.V. gratefully acknowledges support from the Wenner-Gren Foundation through fellowships UPD2018-250 and UPD2019-0076. VGStudio Max (Volume Graphics, Germany) and the Porosity/Inclusion Analysis module were funded by the Vetenskapsrådet through grants 2015-04335; 2019-04595 to S.S. We thank R. DePalma for providing guidance in the field and access to the specimens. We acknowledge the ESRF for provisioning beamtime at BM05. We thank V. Fernandez and K. Chapelle for their assistance with the segmentation in VGStudio; B. Lacet for help with the preparation of the thin and thick sections; M. Hagen for the use of her sedimentology laboratory and the microbalance for weeks in a row; F. Peeters for assistance in photographing the thin sections while sharing his thoughts on the project; and P. Ahlberg for his advice, labelling of the paddlefish bones, fruitful discussions and invaluable consultation.
Funding
Open access funding provided by Uppsala University.